Overview
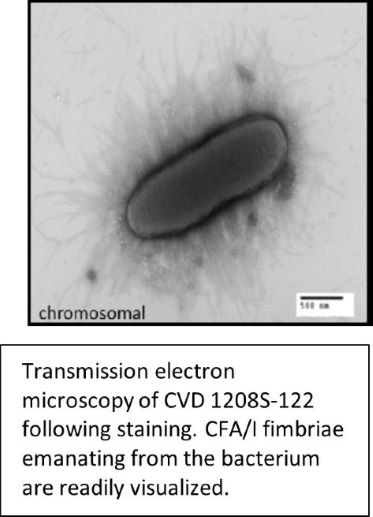
UMB’s optimized Shigella-ETEC vaccine is designed to provide enhanced & essential protection against the clinically important species & serotypes of Shigella and Enterotoxigenic Escherichia coli (ETEC), the major causes of dehydrating diarrheal illness among children under 5 years old in low to middle-income countries, as well as leading causes of travelers’ diarrhea. These pathogens are typically contracted from contaminated food or drinking water, and together caused ~ 20% of all diarrheal disease deaths (> 260,000) in 2016 (Kahlil et al. 2018 The Lancet). Both pathogens are also designated serious threats by the CDC due to increasing multidrug resistance leading to fewer options for therapeutic intervention. UMB’s optimized multivalent vaccine strategy combines 5 to 6 strains of live attenuated Shigella, representing the prevalent species and serotypes found in clinical isolates, and with each Shigella strain engineered to express key ETEC antigens. Engineered deletions in guaBA biosynthetic pathway genes and genes encoding enterotoxins have previously resulted in safe and effective Shigella and Shigella-ETEC candidate vaccines (DeLaine et al. 2016; & Kotloff et al. 2007 Hum Vaccin). Dr. Barry & collaborators have advanced the lead component of their multivalent vaccine “CVD 1208S-122” to a Phase 1 clinical trial. CVD 1208S-122 is live, attenuated S. flexneri 2a expressing ETEC antigens CFA/I and LT (see Fig). Another advanced component of UMB’s vaccine is plasmid-stabilized S. sonnei strain “CVD 1233-SP” (expressing ETEC antigens), which is proven protective against challenge in a guinea pig model (Pilla et al. 2021).
UMB’s vaccine design is informed by our team’s seasoned clinical vaccine development experience, and by global epidemiological data from the “Global Enteric Multicenter Study” (GEMS), a 2011 – 2014 consortium led by UMB’s Center for Vaccine Development. The GEMS was the most comprehensive study of childhood diarrheal diseases ever conducted in developing country settings (> 22,500 children). Several Shigella serotypes were found to be of clinical significance, including the lesser-known S. flexneri 1b and 7a (Livio et al. 2014). Inclusion of these serotypes in our multivalent vaccine is predicted to expand coverage of all Shigella to ~90%. The GEMS also revealed a previously under-recognized ‘minor’ fimbrial antigen from ETEC (“CS14”) as significant in children with moderate-to-severe diarrhea. Inclusion of CS14 in our vaccine is predicted to expand the breadth of ETEC coverage from 64% to 84% (Vidal et al. 2019)
Applications
A broad-spectrum Shigella-ETEC vaccine is expected to be a significant market opportunity, given the high unmet global need amid the current lack of a commercial vaccine, as well as the prevalent disease incidence among travelers. Another candidate Shigella-ETEC vaccine, composed of a single vector strain, published Phase 1 clinical results in 2022 (“ShigETEC”, Eveliqure). It’s anticipated that UMB’s broad-spectrum vaccine will provide significantly enhanced protection over other vaccines.
Advantages
- Rational design
- Broad-spectrum protection
- Clinical-stage development
Stage of Development
A Phase 1 clinical trial (UMB-sponsored, with NIH funding) is enrolling patients (NCT04634513) to test the lead component of our multivalent Shigella-ETEC vaccine, “CVD 1208S-122. The other components of UMB’s multivalent vaccine have been fully characterized and two have been tested in a guinea pig model, with promising safety & immunogenicity.
Licensing Potential
Available for licensing and collaborative R&D
Contact Info
Office of Technology Transfer
620 W Lexington St., 4th Floor
Baltimore, MD 21201
Email: [email protected]
Phone: (410) 706-2380