Overview
There is currently no reliable, durable mitral valve repair option for patients with Functional Mitral Regurgitation (FMR). Replacing the mitral valve with a prosthetic tissue or mechanical valve is currently the most durable approach to the treating FMR. However, this procedure has a high perioperative mortality risk and carries the risk of thromboembolism and prosthetic valve infection and degeneration of bioprosthesis. Mitral valve replacement also requires anticoagulation of the mechanical valves. Another approach for the 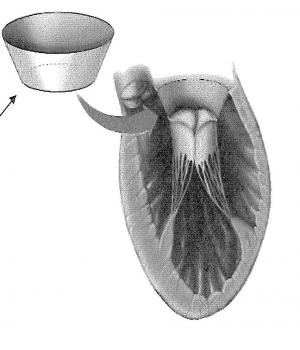 treatment of FMR is restrictive mitral annuloplasty (RMA). Although there are clear benefits to mitral valve repair over replacement, this approach results in a very high rate of recurrence of mitral regurgitation (~60% in two years). Use of a device like the MitraClip® is common and requires a surgical approach (the "Alfieri" stitch) that is known to be only variably effective. Results from treatment of FMR with the MitraClip® have been suboptimal, and a substantial number of patients have either residual or recurrent mitral regurgitation. Dr. Gammie has developed a novel device and method for the treatment of FMR that involves the apical translocation of the mitral valve within the ventricle. The technique should have several advantages over current FMR treatment approaches.
treatment of FMR is restrictive mitral annuloplasty (RMA). Although there are clear benefits to mitral valve repair over replacement, this approach results in a very high rate of recurrence of mitral regurgitation (~60% in two years). Use of a device like the MitraClip® is common and requires a surgical approach (the "Alfieri" stitch) that is known to be only variably effective. Results from treatment of FMR with the MitraClip® have been suboptimal, and a substantial number of patients have either residual or recurrent mitral regurgitation. Dr. Gammie has developed a novel device and method for the treatment of FMR that involves the apical translocation of the mitral valve within the ventricle. The technique should have several advantages over current FMR treatment approaches.
The proposed device allows for the translocation of the patient’s mitral valve within in the ventricle. The positioning of the mitral valve at the apex of the patient’s heart compensates for the geometric issues of FMR, such as annular dilation, annular flattening, leaflet tethering, and increased interpapillary distances. The device reduces mitral regurgitation by improving the position of the mitral valve, increasing coaptation surface area between the leaflets, and decreasing the required amount of leaflet tethering. The technique improves the likelihood of a durable repair and provides for an effective and lasting treatment of FMR. The device can be used alone or in combination with other devices and methods, including the placement of a circumferential band around the papillary muscles and the use of artificial tethers.
Stage of Development
This surgical technique has been tested in acute and chronic ovine models.
Licensing Potential
There are approximately six million FMR patients in the United States and nearly as many in Europe. FMR exacerbates heart failure-related symptoms and is associated with increased hospitalization and mortality rates.
Contact Info
Office of Technology Transfer
620 W Lexington St., 4th Floor
Baltimore, MD 21201
Email: [email protected]
Phone: (410) 706-2380